



Металл ртуть (Hg) - silver metal (Kip t=357 °C). Under normal conditions - the heaviest of all known liquids (molten metal).
Metal mercury weighs 13.6 kg liter at 20 °C. Ordinary glass jar could break up under the weight of the mercury. Therefore, large amounts of metal mercury stored in a special thick-walled glass containers or metal containers. Low melting point mercury (-39 °C) due to the atoms of mercury (Hg) save their valence electrons and struggle to provide them in "General use". Thus, the crystal lattice of mercury, is unstable. By the way, so mercury bad conductor of heat and electric current.
Many metals (sodium metal, zinc, cadmium and others) are easily dissolved in mercury (take in chemical reactions) with the formation of amalgam is liquid or solid alloys. Before this property of mercury used for gettig a mirrors by coating on the glass amalgam tin. The ability of mercury to dissolve metallic sodium and metallic potassium is used in electrolytic production alkali.
liquid mercury evenly expands when heated, so it is filled thermometers.
Mercury, unlike their "neighbors" in the subgroup of the periodic table, is a light (inactive) metal. Dissolve it in Aqua Regia or concentrated nitric acid:
Hg+4HNO3→ Hg(NO3)2+2NO2+2H2O.
Almost all metals, except pure gold, silver and platinum, can displace mercury from solutions of its salts. Metal mercury is one of the few metals not forming hydroxides. Hydroxide mercury (II) when it formed the detaches water and turning into mercury oxide:
Hg(NO3)2+2NaOH→ HgO+Н2О+2NaNO3.
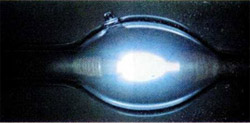
If to fill the glass tube by mercury vapor and attach to the ends of the tension, there arise charged particles: Hg+ e-. When ions mercury rush towards the negatively charged electrode, and the electrons to the positive. When the reverse process is formed by excited atoms that lose energy by emitting a quantum of light. A large part of the light is in the ultraviolet part of the spectrum.
Mercury vapor lamp is also called quartz, because the tube is made from quartz, through which can pass the ultraviolet light. These lamps are used in laboratories for photochemical reactions, in the banking industry to recognize the authenticity of banknotes and in the artificial tan. Ultraviolet rays are harmful for the eyes, so a mercury lamp is not suitable for lighting. However, the glass bulb may be coated inside with luminophore is a substance that absorbs ultraviolet light and emits visible light. The so-called fluorescent lamp, the light from which the parameters corresponds to daylight (fluorescent).
Oddly enough, mercury if ingestion is almost no threat. The described cases, when people for suicide drank mercury and even it was injected intravenously, but nothing not occurred with them. The perfect antidote when ingested salts of mercury is a raw egg. It consists of almost entirely of protein, containing a number of sulfhydryl groups, which are strongly associated mercury. However, to take the antidote you need very quickly - while mercury is not absorbed into the blood.
Mercury is one of the ten most dangerous poisons. Especially toxic carbon compounds with mercury C-Hg, for example ion methylmercury H3C-Hg+. Any inorganic compound mercury (II), trapped in the body, under the action of methylcobalamin (vitamin b12) - substances, the giver CH3group, is transformed into methylmercury ion. All connections methylmercury are soluble and therefore easily penetrate from cell membranes.
Toxicity of mercury due to the fact that it forms a very strong relationship with sulfur. If the protein is an enzyme formed a relationship with mercury, its shape changes and it loses its biological activity. Trapped in the body of mercury is practically not removed from it, and gradually accumulates. Most of the mercury contained in the bodies of predatory fish and birds, their eating. Fish accumulate mercury as methyl mercury, even from poorly mercury contaminated waters. By eating fish from these waters people get mercury in the tens or even hundreds of times more than those who do not eat this fish.