Corrosion protection
Corrosion was almost 1/3 of newly commissioned metal. Part of it is melted and returns to the industry. But still, 10% of the total weight - are lost forever. The destruction of individual metal parts may entail the destruction of entire mechanisms, creating an emergency situation. In this connection, creating a metal objects, devices, mechanisms, greater attention should be paid to corrosion protection. A radical method for corrosion protection against searchind corrosion resistant materials for aggressive environment. Completely replace metals on non-metallic objects - is impossible.
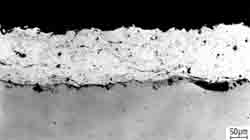
Corrosion protection allows timely and reliable to isolate the metal from corrosive environments. To protect the metal against destruction with creating on its surface a protective film - coating. The way of creating protective films are different. For example, a corrosive metal coated with a layer of another metal which is not destroyed under the same conditions. As coatings are used non-metallic coatings, organic materials - film high-polymeric substances, paints, linseed oil, as well as compositions of high polymer and inorganic pigments.
Of particular importance are films of metal oxides obtained with the action of oxygen or suitable oxidizing agents (nitric acid HNO3, potassium dichromate K2Cr2O7 and others.) on the surface of metals. Often, these oxide films are formed on the surface of metals even just in contact with the air, making a chemically-active metals (aluminum, zinc) is corrosion resistant. Such is the role of protective nitride coatings, formed with the action of nitrogen or ammonia to the surface of some metals. Artificial oxidation, nitriding, phosphating are good protections of metals against corrosion.
Electrochemical corrosion protection
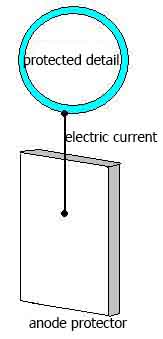
Electrochemical corrosion protection metallic objects based on a change of potential of the protected metal and is not related to isolation of the metal from the corrosive environment. It is cathodic protection. It is also called anode protection.
Cathodic protection - is that the protected design "A" in the electrolyte medium (e.g., soil water) is attached to the cathode (negatively charged electrode) of a source of electricity. Design of the protected becomes the cathode. In the same hostile environment put a piece of old metal (rail or beam) to be attached to the anode external source of electricity. In the process of corrosion, this piece of old metal becomes the anode and gets destroyed.
Protector corrosion protection different from cathodic protection becouse for its uses a special anode - protector, which used metal is more active than the protected metal (aluminum, zinc). Protector connected to the protected design with conductor of electrical current. In the process of corrosion protector is an anode (positively charged electrode) and destroyed, thereby protecting from destruction of our protected design.
Consider the corrosion process, damage to the metallic protective coating.
1. If the metal is coated with a less active metal. For example, tin (Sn) covers the iron (Fe) and quite resistant against the action of dilute acids. In case of damage to such coating occurs a galvanic elemet in that the electron is transferred from iron to tin, that is, the anode is iron (it dissolves and breaks), and the cathode - tin (remains unchanged).
2. If the metal coating with more active metal. For example, coatings of iron (Fe) with zinc (Zn). After mechanical damage to the zinc coating occurs a galvanic elemett in that iron serves as the cathode (not dissolved), and zinc is anode. In this case the iron will not be destroyed until then destroyed zinc.
Of these cases, we can conclude that the reliable protective coating that metal is more active than the protected metal.
Corrosion protection
Corrosion inhibitors
To slow corrosion of metal objects in a hostile environment adds a substance (often organic), called >corrosion inhibitors (retarders of oxidation of the metal). This method is successfully used when it is necessary to protect metal from corrosion acid. Inhibitors are widely used in the purification of steam boilers from scale, for removing scale from the waste parts and then storage and transportation hydrochloric acid in a steel container.
As organic inhibitors is used thiourea (chemical name sulfide-diamide carbon C(NH2)2S ), diethylamine, methenamine (CH2)6N4) other derivatives of amines.
As inorganic inhibitors is used silicates (compounds of metals with silicon Si), nitrites (compounds of nitrogen N), dichromats of alkali metals etc. Sometimes with the removal of oxygen from water can also be achieved reducing the corrosive properties of water. And do this with filtering water through a layer of iron filings!
Cleaning of steel items
To remove the rust with mechanical means is impossible. Therefore often used solutions containing strong chemical reagents - acids, bases, etc. With the elimination of rust is the effect of the surface protection from external influences. Slightly soiled and rusty items for a few hours soaked in gasoline, and then dirt and rust removed with a cloth soaked with gasoline or wire brush for deep rust. Steel items can be cleaned with a paste the composition of which follows: engine oil - 650 grams, paraffin - 150g, very fine pumice stone - 200 grams or naphtha - 270g, abrasive powder - 450 grams, aluminum powder - 40 g
Cleaning of non-ferrous metals
In a porcelain vessel melt 100 grams of paraffin, 200 grams of the olein, 200 g of sheep fat. The resulting mixture added 500 g of chalk powder and stirred until complete homogenization.
Cleaning silver
In a porcelain or enameled vessel in 100 ml of warm water dissolve 300 g white soap, 150 g of oxalic acid, 150 g of urea calcium.