Why blacken fruit knives?!

Did you know for example, why blacken fruit knives?! If you add some fruit juice to solution of iron salts (iron salts solution can be got easily at home, if to put, for example, a nail to copper sulphate, for half an hour or a few buttons, paper clips), the liquid will darken. We will get the weak solution of the ink. Fruits contain tannic acid and form ink with the salt of iron. In order to get a solution of iron salts at home, dip the nail into a solution of copper sulphate, and wait ten minutes. Then drain the greenish solution. The resulting solution of ferric sulfate (FeSO4) can be used in reactions.
Tea also contains tannic acid. A solution of iron salts, added to a weak solution of tea, change its color to black. It is not recommended to brew the tea in a metal kettle!
Chemical reactions with food salt
Sometimes iodides of sodium or potassium add to food salt. It because iodine is included in various enzymes of the our body, and its deficiency impairs functioning of thyroid gland.

Discover a supplement simply. To cook starch paste: quarter of a teaspoon of starch diluted in cold water, heat to boiling, boil five minutes and cool. The starch paste is considerably more sensitive to iodine than dry starch. Next a third part of a teaspoon of salt dissolved in a one teaspoon of water, then to the resulting solution add a few drops of vinegar (or half a teaspoon of vinegar), half a teaspoon of hydrogen peroxide and then after two or three minutes a few drops of starch paste. If salt was iodised, the peroxide will displace iodine:
2I-+ Н2О2+2СН3СООН→ I2+2Н2О+2СН3СОО-,
which will paint the starch to blue. (Experience will not be if the iodination of salt was used KClO3 instead KI). You can do experience with copper sulphate and food salt. There will not be none of the above reactions. But the reaction is beautiful... When mixed copper sulphate and food salt see the formation of a beautiful green solution of sodium tetrachlorocuprate Na2[CuCl4]
Interesting experiments with potassium permanganate:
Dissolve in the water some crystals of potassium permanganate and wait some time. You will notice that the crimson colour of the solution (due to the presence of the permanganate ions in the solution) gradually becomes paler, and will disappear, but on the walls of the vessel you can see a brown manganese oxide (IV):
4КMnО4+2Н2О→ 4MnO2+4КОН+3О2
The dishes in which you did the experience, easily clear with a solution of citric or oxalic acid. These substances restore the manganese to oxidation state +2 and transfer it to water-soluble complex substances. Solutions of potassium permanganate can persist for years in dark flasks. Many believe that potassium permanganate is highly soluble in water. In fact, the solubility of this salt at room temperature (20 °C) is only 6.4 g per 100 g of water. However the solution is so vivid coloring that seems to be concentrated.
If you heat potassium permanganate to 200 0C, the potassium permanganate will turn to dark green manganate of potassium (К2MnO4). This releases a large amount of pure oxygen that can be collected and used for other chemical reactions. A particularly fast solution of potassium permanganate deteriorates (breaks down) in the presence of reducing agents. For example, the reducing agent is ethanol C2H5OH. Reaction of potassium permanganate with alcohol proceeds as follows:
2КMnO4+3C2H5OH→ 2KOH+2MnO2+3CH3CHO+2H2O.
Washing agent of potassium permanganate:
To get a homemade washing agent, it is necessary to mix the potassium permanganate with the acid. Of course, not all acids. Some acids can be oxidized; in particular, if we take hydrochloric acid, it will emits toxic chlorine:
2КMnO4+16HCl→ 2MnCl2+5Cl2+2KCl+8Н2О.
So it often get in the laboratory. Therefore, for our purposes it is better to use a diluted (about 5%) sulfuric acid. You can replace it with dilute acetic acid — vinegar. Take about 50 ml (? cup) of the acid solution, add 1-2 g of potassium permanganate (on the tip of a knife) and mix thoroughly with a wooden stick. Then wash it with water and tie it to the end of a piece of foam sponge. This "brush" quickly, but gently squash the oxidation mixture on the contaminated polluted place. Soon the fluid changes color to dark red and then - brown. The oxidation reaction began.
Here it is necessary to make a few comments. It is necessary to work very carefully so the mixture does not get on hands or clothing; it would be good to put oilcloth apron. And you should not hesitate because the oxidation mixture is very caustic and "eats" even the foam. After using the foam brush can be put in a jar of water, rinse and throw away. During then cleaning can cause unpleasant smell produced by the products of incomplete oxidation of organic pollutants on the porcelain and the acetic acid, so the room should be aired.
After 15-20 minutes we'll take the browned mixture with water. On the place appear brown spots, don't worry: the product recovery of the permanganate — manganese dioxide MnO2 is easy to remove, restoring insoluble manganese (IV) to water-soluble salts of manganese.
But when the potassium permanganate interacts with concentrated sulfuric acid, it forms the oxide of manganese (VII) Mn2O7 is an oily dark-green liquid. This is the only liquid under normal conditions the oxide of the metal (T melt=5,9°C). It is very volatile and easily explodes with a slight heating (t=55°C) or concussion. Mn2O7 is stronger oxidizing agent than KMPO4. When contacted, it ignite many organic substances such as ethyl alcohol. It is one of the ways to light a spirit-lamp, with no matches!
Interesting experiments with hydrogen peroxide
Hydrogen peroxide can be an oxidizing agent (this property is widely known), and the reducing agent! In the latter case, it reacts with substances-oxidizing agents:
Н2О2-2е=2Н++О2. Manganese dioxide is such a substance. Such reactions chemists call "reducing decomposition of hydrogen peroxide".
Instead of drugstore peroxide you can use special tablet — compounds of hydrogen peroxide with urea CO(NH2)2•Н2О2. This is not a chemical compound, as between molecules of urea and hydrogen peroxide there is no chemical bonds; molecules of H2O2 as would be included in long narrow channels in crystals of urea and can't come out until the substance is not dissolved in water. Therefore, these substances are called channel inclusion substances. One tablet corresponds to 15 ml (a tablespoon) of 3% solution of H2O2. To get a 1% solution of H2O2 taking double dose and 100 ml of water.
Using manganese dioxide as the oxidizing agent for hydrogen peroxide, you need to know: MnO2 is a good catalyst for the reaction of decomposition of H2O2 to water and oxygen:
2Н2О2→ 2Н2О+О2.
And if you just handle the place (sink) with a solution of H2O2, he instantly "boil", producing oxygen, and the tarnish will remain, because the catalyst must not be wasted during the reaction. To avoid catalytic decomposition of H2O2, needs acidic environment. Here too the vinegar.Ppharmacy peroxide heavily dilute with water, add a little vinegar to this mixture and clean the place (sink). Magic! A dirty-brown surface will be white like a new. And the miracle happened with accordance reaction
MnO2+Н2О2+2Н+→ Mn2++2Н2О+О2.
It only remains to wash out soluble salt of manganese with water. The same way you can use to clean soiled aluminum pan: in the presence of strong oxidizers on the surface of the metal forms a durable protective film of oxide, then protects it from dissolution in acid. But cleaned with this method of enamel products (pots, tubs) not recommend: acidic environment slowly destroys the enamel. For removing plaque MnO2 can also used aqueous solutions of organic acids: oxalic, citric, tartaric. Acid create in an aqueous solution sufficiently acidic environment.
Interesting experiments

"Gold" in the flask
Of course, gold is not present, but the experience is beautiful! For chemical reactions we need a soluble salt of lead (lead acetate (CH3COO)2Pb - salt formed with dissolving lead in acetic acid) and salt of iodine (e.g. potassium iodide KI). Lead acetate can be got at home, dropping a piece of lead in acetic acid. Potassium iodide is sometimes used for etching electronic boards.
Potassium iodide and lead acetate are two liquids, the appearance is no different from water.
let's Start a reaction: to a solution of potassium iodide add a solution of acetate of lead and watch formation of a golden yellow precipitate of lead iodide PbI2, - impressive! The reaction proceeds as follows:
(CH3COO)2Pb+KI→ CH3COOK+PbI2
Entertaining experiments with liquid glass
Stationery glue is liquid glass or its chemical name "sodium silicate" Na2SiO3 We can say also it is a sodium salt of silicic acid. If add glue the silicate solution of acetic acid, the precipitate will fall out as insoluble silicic acid, hydrated silicon oxide.
Na2SiO3+2СН3СООН→ 2CH3COONa+H2SiO3.
The resulting precipitate H2SiO3 can be dried in the oven and diluted with solution of water-soluble ink. As a result the ink cover the surface of silicon oxide, and then you can not wash it. This process is called adsorption (lat. ad — "to" and sorbeo — "absorb")
Another beautiful entertaining experience with liquid glass. We will need copper sulphate CuSO4, sulphate of Nickel NiS04, ferric chloride, FeCl3. Do chemical aquarium. In a tall glass jar with silicate glue, diluted with water, simultaneously pour out from two glasses the aqueous solutions of Nickel sulfate and ferric chloride. In the jar gradually grow silicate "algae" yellow-green colours that intertwine, fall from the top to down. Now add in a jar drop by drop a solution of copper sulfate, populate aquarium "sea stars". Algae growth is a result of crystallization of hydroxides and silicates of iron, copper and nickel, these are formed as a result of exchange reactions.
Interesting experiments with iodine
Add the iodine to a few drops of hydrogen peroxide, H2O2 and mix up. After some time we will have a highlighted black precipitate. This is crystalline iodine is poorly water soluble substance. Iodine drop out faster if the solution is slightly warm with hot water. The peroxide is needed to oxidize potassium iodide KI (it is added, to increase the solubility of iodine). With the poor solubility of iodine in water is another its ability - to be extracted from water by liquid composed of nonpolar molecules (oil, gasoline, etc.). In a teaspoon of water add a few drops of sunflower oil. Mix up and see that the oil is not miscible with water. Now if there is to drip two or three drops of tincture of iodine and shake, the layer of oil will turn dark brown color, and the water layer is pale yellow, i.e. most of the iodine will pass into the oil.
Iodine is very corrosive. To verify this, a few drops of iodine put on a metal surface. After some time the liquid becomes discolored, and the metal surface will have a stain. The metal is reacted with iodine with formation of iodide salt. On this property of iodine based one way of drawing on metal.
Color entertaining experience with ammonia
Substance "ammonia" we mean an aqueous solution of ammonia. In fact, ammonia is a gas that when dissolved in water and forms a new class of chemical compounds - "base". With the base and we'll have experiment. Entertaining experience can be done with a solution of ammonia (liquid ammonia). Ammonia reacts with copper ions and form color substance. Take a bronze or copper coin with a dark patina and fill it with ammonia. Immediately or after a few minutes the solution will turn blue. It is with the action of the oxygen in the air the copper formed a complex substance.
2Cu+8NH3+3Н2О+О2→ 2[Cu(NH3)4(H2O)2](OH)
Interesting experiments: liming
The liming is a chemical reaction between the oxide of calcium (Cao - quicklime) and water. It proceeds as follows:
CaO + H2O→ Ca(OH)2.
Calcium hydroxide (Ca(OH)2) is also called milk of lime . If in a solution of calcium hydroxide to miss carbon dioxide (or to breathe into a tube through the solution), you'll get a white insoluble precipitate of calcium carbonate:
Ca(OH)2 + CO2→ CaCO3 + H2O.
This reaction is also high quality reaction for calcium ions Ca+ in solution. The resulting substance, calcium carbonate is well-known chalk (lime, crayons).
Ignition temperature in air of some complex substances, 0С:
- Acetone - 465
- Gasoline - 350
- Ethanol - 404
- Diethyl ether - 164

The beginning of the reaction between magnesium and iodine. Emissions of iodine vapor
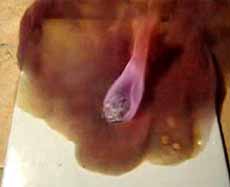
Iodine vapour and intense oxidation of magnesium
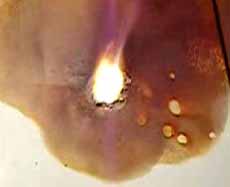
The last stage - formation of iodide of magnesium