Chemical experiments with bromine and aluminum
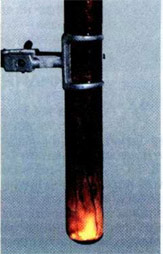
If a few milliliters of bromine put in a test tube of heat-resistant glass and carefully drop in it a piece of aluminum foil, then after some time (it needs to order the bromine passes through the oxide layer) starts a violent reaction. Aluminum melts because of the heat and get the form of a small fiery ball rides on the surface of the bromine (density of liquid aluminum is less than the density of bromine), rapidly decreasing in size. The tube is filled with bromine vapors and white smoke, that is consisting of very small crystals of aluminum bromide:
2Al+3Br2→ 2AlBr3.
It is also interesting to watch the reaction of aluminum with iodine. Mix in a cup a small amount of powdered iodine with aluminium powder. While the reaction is not observable: with the absence of water, it is very slowly. Using long pipette to drip to the mixture of a few drops of water, and the reaction is going more vigorously - with the formation of the flame and the release of violet vapor of iodine.
Chemical experiments with gunpowder: How the gunpowder explodes!

Smoky or black, the gunpowder is a mixture of potassium nitrate (potassium nitrate is KNO3), sulfur (S) and carbon (C). It ignites at a temperature of about 300 0C. Powder may explode with shock. It consists of an oxidizer (nitrate) and reductant (coal). Sulphur is also a reducing agent, but its main function is to bind potassium in strong compound. When burning gunpowder reaction goes:
2KNO3+3C+S→ K2S+N2+3CO2,
- to produce a large amount of gaseous substances. This explains the use of gunpowder in military: formed with the explosion and expanding from the heat of the reaction gases push the bullet from the gun. You can fill the formation of sulfide
potassium is easily, sniffing the gun after. It smells of hydrogen sulfide - product of the hydrolysis of potassium sulfide.
Chemical experiments with nitrate: a fiery inscription
Spectacular chemistry experiment can be made with potassium nitrate. Recall, that nitrates is complex substances - a salts of nitric acid. In this case we need potassium nitrate. Its chemical formula KNO3. On a sheet of paper draw the outline, figure (to great effect let the lines do not intersect!). Prepare a concentrated solution of potassium nitrate. For information: in 15 ml of water is dissolved 20 g of KNO3. Then with a brush impregnated paper along the drawn contour, do not leave gaps and free spaces. let the paper dry. Now you have to touch with burning splinter to any point on the contour. Immediately will be the "spark" that will move slowly along the contour of the drawing until close it completely. What is happens: potassium nitrate decomposes according to the equation:
2KNO3→ 2 KNO2 +O2.
So, KNO2 +O2 - salt of nitrous acid. With oxygen released the paper chars and burns. For greater effect, the experience may be performed in a dark room.
Science experiment dissolving the glass in hydrofluoric acid

in hydrofluoric acid
Indeed, the glass dissolves easily. Glass is a very viscous liquid. The glass can dissolve, you can confirm with doing the following chemical experiments. Hydrofluoric acid - this acid is formed with dissolving hydrogen fluoride (HF) in water. For greater clarity, take a thin glass and attach the sinker to it. Glass with sinker dip in a solution of hydrofluoric acid (look at the picture). When the glass dissolves in the acid, the sinker will fall to the bottom of the flask.
chemical experiments with smoke emission

with smoke emission
(ammonium chloride)
Let do a beautiful experience to get a dense white smoke. To do this, we need to prepare a mixture of potash (potassium carbonate K2CO3) with ammonia solution. Mix the reagents: potash and ammonia. To the resulting mixture add a solution of hydrochloric acid. The reaction will begin at the moment when the flask with the hydrochloric acid will be presented to the bulb with ammonia. Add the hydrochloric acid to the ammonia solution and watch the formation of a dense white vapor of ammonium chloride with chemical formula NH4Cl. Chemical reaction between ammonia and hydrochloric acid is as follows:
HCl+NH3→ NH4Cl
Chemical experiments: Glow solutions

As noted, glow of solutions is a sign of a chemical reaction. It will do an experience with glow of solution. For the reaction, we need a solution of luminol, hydrogen peroxide solution H2O2 and crystals of blood red salt K3[Fe(CN)6]. Luminol is a complex organic substance with formula C8H7N3O2. Luminol soluble in some organic solvents, does not dissolve in water. Glow reaction of luminol you can see with some oxidizing agents in alkaline medium.
So, let's start: add a solution of hydrogen peroxide to luminol, then to the resulting solution add a crystals of red blood salt. For greater effect, try to experience in a dark room! As soon as the blood red crystals touch the salt solution, will be immediately noticeable cold blue glow that indicates the chemical reaction. Glow when a chemical reaction is called chemiluminescence
Another chemistry experiment with glowing solutions:
Yo do it we need: hydroquinone (previously used in photographic equipment), potassium carbonate K2CO3 (also known as "potash"), a pharmaceutical solution of formalin (formaldehyde) and hydrogen peroxide. Dissolve 1 g of hydroquinone and 5 g of potassium carbonate K2CO3 in 40 ml of formalin (aqueous formaldehyde). Pour this reaction mixture in a large flask or bottle with capacity more or one litre. In small bowl prepare 15 ml of concentrated hydrogen peroxide solution. You can use a tablet of urea - the of hydrogen peroxide with urea (urea does not hurt the experience). For greater effect, go in a dark room when your eyes adjust to the dark, pour the solution of hydrogen peroxide in a large bottle with hydroquinone. The mixture will start to foam (so take large bottle) and there will be a distinct orange glow!
A chemical reactions with luminescence happen not only then oxidation. Sometimes illumination occurs during crystallization. The simplest way of monitoring - food salt. Dissolve the food salt in water, but take it as much as the salt remain on the bottom of the glass. This saturated solution pour into another glass and add dropwise to this solution concentrated hydrochloric acid. Salt begins to crystallize, the solution will take a sparks. The most beautiful, if it experience set in the dark!

Chemical experiments with chromium and its compounds
Colored chrome!... Coloring salts of chrome can easily change from purple to green and vice versa. Will have the chemical experiments: dissolve in water are some purple crystals of chromium chloride CrCl3*6H2O. With boiling the purple solution of this salt becomes green. Evaporation of the green solution forms a green powder of the same composition as that of the original salt. But if cooled to 0 0C a green solution of chromium chloride saturate with hydrogen chloride (HCl), color it will become purple again. How to explain the phenomenon? It's rare in inorganic chemistry example of isomerism - the existence of substances with the same composition but different structure and properties. In the purple salts of chromium atom is associated with six water molecules and the chlorine atoms are counterions: [Cr(H2O)6]Cl3, but in the green chloride of chromium they change places: [Cr(H2O)4Cl2]Cl*2H2O. In acidic medium dichromate are strong oxidizing agents. The products of their reduction are ions Cr3+:
K2Cr2O7+4H2SO4+3K2SO3→ Cr2(SO4)3+4K2SO4+4H2O.

dichromate - (red)
At low temperature of the formed solution was able to identify purple crystals hromokalievyh alum KCr(SO4)2*12H2O. Dark-red solution got then add concentrated sulfuric acid to a saturated aqueous solution of potassium dichromate is called a "bichromate". In laboratories it is used for cleaning and degreasing chemical glassware. Glassware carefully rinsed with bichromate, which is not poured down in the sink and used repeatedly. In the end the mixture becomes green - whole chromium in it solution already change form to Cr3+. A particularly strong oxidizing agent is oxide (VI) - CrO3. It can be used to light a spirit lamp without matches: enough to touch the alcohol-soaked wick with a stick with a few crystals of the substance. During the decomposition of CrO3 can be got dark-brown powder of chromium oxide (IV) CrO2. It has ferromagnetic properties and used in magnetic tapes some cassettes. The body of an adult contains about 6 mg chromium. Many substances of this element (particularly chromates and dichromates) are toxic and some are carcinogenic i.e. can cause cancer.
Chemical experiments: reduction properties of iron

This type of chemical reaction refers to the redox reactions. For the reaction we will need to dilute (5%) aqueous solutions of ferric chloride(III) FeCl3 and the same solution of potassium iodide KI. So in one beaker is poured a solution of ferric chloride(III). Then add to it some drops of solution of potassium iodide. Watch the color change of the solution. The liquid will acquire a red-brown color. In the solution will occur following chemical reaction:
2FeCl3 + 2KI→ 2FeCl2 + 2KCl + I2
KI + I2 = K[I(I)2]

Another chemical experience with iron compounds. To do it we need a dilute (10-15%) aqueous solutions of iron(II) sulphate FeSO4 and ammonium thiocyanate NH4NCS, bromine water Br2. Let's start. In one flask pour a solution of ferrous sulfate(II). In it flask add 3-5 drops of a solution of ammonium thiocyanate. Notice that there are no signs of chemical reactions. Of course, the cations of iron(II) don't form colored complexes with thiocyanate-ions. But now in this flask add bromine water. And now iron ions "gave himself" and the solution painted in blood red. This is a typical reaction of ion (III)-valent iron with the thiocyanate ions. Here's what was in the flask:
6FeSO4 + 3Br2→ 2Fe2(SO4)3 + 2FeBr3
Fe(H2O)6]3+ + n NCS- [Fe(H2O)6-n(NCS)n](n-3) - + n H2O
Chemical experiment for the dehydration of sugar with sulfuric acid
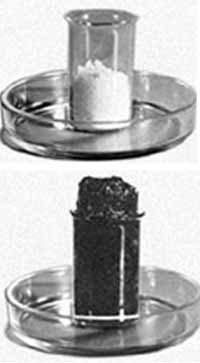
Concentrated sulphuric acid dehydrates sugar. Sugar is a complex organic substance with formula C12H22O11. How it works? The powdered sugar is put in high glass beaker, moisten with a little water. Then add to the sugar a little concentrated sulfuric acid, gently and quickly mix with a glass stick. Stick leave in the glass with the mixture. After 1 - 2 minutes the sugar starts to blacken, swell, loose mass. The mixture is strongly heated and a little smoke. During this chemical reaction the sulfuric acid takes away a water from sugar and also partly turns it into coal.
C12H22O11+2H2SO4(conc.)→ 11C+CO2+13H2O+2SO2
Water, that is released during this chemical reaction, mainly absorbed with the sulfuric acid (sulfuric acid "greedy" absorbs water) with the formation of hydrates, hence strong heat. And carbon dioxide CO2, is got with the oxidation of sugar and sulfur dioxide SO2 applyvalues lift the mixture up.
Chemical experience with the disappearance of the aluminum spoon
Do another funny chemical reaction: for this we need aluminum spoon and mercury nitrate (Hg(NO3)2). So, take a spoon and scrape it with a fine-grained emery paper, then clear it with acetone. Dip a spoon for a few seconds in a solution of mercurous nitrate (Hg(NO3)2) (but remember, that mercury substansec are poisonous!). Then the surface of aluminum spoons will grey, the spoon should be removed, wash with boiled water, dry (but not wiping). After a few seconds a metal spoon will turn into white fluffy flakes and soon it will only grey ashes. What happened:
Al + 3 Hg(NO3)2 = 3 Hg + 2 Al(NO3)3.
At the beginning of the reaction on the surface of the spoon a thin layer of aluminum amalgam (an alloy of aluminum and mercury). Then the amalgam turns into a white fluffy flakes of aluminum hydroxide (Al(OH)3). Consumed in the reaction the metal is replenished with new portions of aluminum, dissolved in mercury. Finally, instead of a shiny spoon on the paper stays white powder Al(OH)3 and tiny drops of mercury. If after solution of mercurous nitrate (Hg(NO3)2) an aluminum spoon immediately put in distilled water, then the surface will appear bubbles of gas and flakes of white color (there will be release of hydrogen and hydroxide of aluminum).