The biological role of proteins is extremely high: they make up the bulk of the protoplasm and nuclei of living cells. Proteins are found in all plants and animals. Amount of proteins in nature can be estimated by the total amount of living matter on our planet: the mass of the protein is approximately 0.01% of the mass of the earth's crust, that is 1016 tons.
Proteins in elemental composition different from carbohydrates and fats: in addition to carbon, hydrogen and oxygen they contain nitrogen. In addition, sulfura is important a permanent part of the protein, and some proteins contain phosphorus, iron and iodine.
Properties of proteins
1. Different solubility in water. Soluble proteins form colloidal solutions.
2. Hydrolysis is the action of solutions of mineral acids or enzymes is the destruction of primary structure of the protein and the formation of a mixture of amino acids.
3. Denaturation - the partial or total disruption of the spatial structure inherent in a protein molecule. Denaturation is activated with:
- high temperature
- solutions of acids, alkalis and concentrated salt solutions
- solutions of salts of heavy metals
- some organic substances (formaldehyde, phenol)
- radiation
The structure of proteins began to study in the 19th century. In 1888 Russian biochemist A. I. Danilevsky has stated a hypothesis about the presence amide bond in the proteins. This idea was later developed by the German chemist E. Fischer and found experimental confirmation in his works. He suggested polypeptide theory of the structure of the protein. According to this theory, the protein molecule consists of one long chain or several polypeptide chains, linked to each other. Such chains may be different lengths.
Fisher held a lot of experimental work with polypeptides. Highest polypeptides containing 15 to 18 amino acids are precipitated from solutions of ammonium sulphate (ammonium alum), that is, exhibit the properties characteristic for proteins. It was shown that the polypeptides are cleaved by the same enzymes as and proteins. When it introduced into the animal organism, it has a same transformations as proteins, and all nitrogen is normally formed in urea (carbamide).
Research in the 20th century, showed that there are several levels of the organization protein molecule.
Our body has thousands of different proteins and almost all of them are built from a standard set of 20 amino acids. The sequence of amino acid residues in the protein molecule is called a primary structure of protein. Properties of proteins and their biological functions are determined by the sequence of amino acids. Work on the elucidation of the primary structure of the protein were first performed in Cambridge University and used the example of one of the simplest protein - insulin. During the post 10 years English biochemist F. Sanger did an analysis of insulin. The analysis revealed that the molecule insulin consists of two polypeptide chains and contains 51 amino acid residue. F. Sanger found that insulin has a molar mass 5687 g/mol and its chemical composition has formula C254H337N65O75S6. The analysis was conducted manually using enzymes that selectively hydrolyze peptide bonds between specific amino acid residues.
Now most of the work to defining primary structure of proteins is automated. Thus primary structure of the enzyme lysozyme was established .
Type "stacking" of the polypeptide chain is called secondary structure. The majority of proteins polypeptide chain coils into a spiral, resembling a "stretched spring" (called "A-helix" or "A-structure"). Another common type of secondary structure - the structure of the folded sheet (called "B - structure"). So, silk protein - fibroin has this structure. It consists of a number of polypeptide chains, which are parallel to each other and interconnected by hydrogen bonds, a number of which makes silk very flexible and tear-resistant.
But, there is virtually no proteins, the molecules of which 100% have the "A-structure" or "B - structure."
The spatial position of the polypeptide chain is called the tertiary structure of the protein. The majority of proteins are globular, because their molecules are folded into globules. This form of protein is supported through links between ions witn different charges(-COO- and NH3+) and disulfide links. In addition, proteins is folded so that hydrophobic hydrocarbon chains are inside globules and hydrophilic are outside.
A method for combining several molecules of protein in one macromolecule is called Quaternary protein structure. An example of such a protein may be hemoglobin. It was found that, for example, for an adult, a molecule of hemoglobin consists of 4 separate polypeptide chains and non-protein parts - heme.
Different structure ofproteins explains the properties. Most proteins are amorphous, insoluble in alcohol, ether and chloroform. In water, some proteins can be dissolved with the formation of colloidal solution. Many proteins are soluble in alkaline solutions, some in the salt solutions, some in dilute alcohol. Crystalline proteins are rare: an example may be the aleurone, found in the castor bean, pumpkin, and hemp. Crystallized also albumin of chicken eggs and hemoglobin in the blood.
When boiled with acids or alkalies, and also under the action of enzymes, proteins are broken down into simpler chemical compounds and form in the end of a chain of transformation mixture A-amino acids. This splitting is called a protein hydrolysis. protein Hydrolysis is great biological significance: in the stomach of an animal or human, the protein is cleaved under the action of enzymes on amino acids. The formed amino acids in next time with enzymes form proteins again, but typical for this organism!
In the products of hydrolysis of proteins in amino acids were found carbohydrates, phosphoric acid and purine bases. Wtit the influence of some factors such as heat, salt solutions, acids and bases, the effects of radiation, shaking, may disrupt the spatial structure of this protein molecule. Denaturation can be worn reversible or irreversible, but in any case, amino acid links, that is, the primary structure remains unchanged. As a result of denaturation the protein ceases to perform its inherent biological function.
For proteins known some color reactions characteristic of detection. When heated urea, biuret is formed, which with the copper sulfate solution with presence alkali gives a purple colour (qualitative reaction for protein. The biuret reaction gives the compounds containing amide group in the protein molecule, this group is present in the protein.
When ksantoproteinovaya reaction the protein acid turns yellow with presence concentrated nitric. This reaction indicates the presence of benzene group in the protein, which includes in amino acids as phenylalanine and tyrosine.
When boiled with an aqueous solution of mercurous nitrate and nitric acid, protein gives a red coloration. This reaction indicates the presence tyrosine in the protein. When tyrosine absence the red staining does not appear.
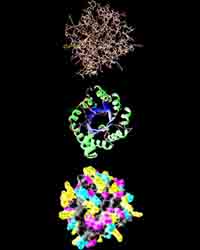
Protein
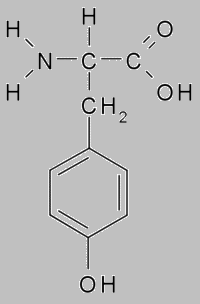
Protein tyrosine
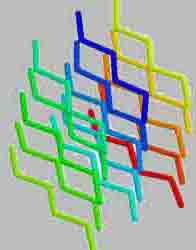
Protein fibroin is a natural silk protein
Complex substances. Water hardness
Properties of acids
Getting acids
Alcohols. Properties of alcohols
Enzymes. The action of enzymes
What is the enzyme. What enzymes are formed