
Complex substances
Complex substances - a chemical substance formed by the combination of several simple substances. In our economy we use complex substances greater and simple substances lesser. Salts oxides,bases or alkali, acids are used by us as a complex substances, and also many organic compounds, such as alcohols, paraffins, aldehydes, etc.
Water
| Your waight | Day norm |
| 18 kg | 0,5 l |
| 27 kg | 0,75 l |
| 36 kg | 1,0 l |
| 45 kg | 1,25 l |
| 54 kg | 1,5 l |
| 63 kg | 1,75 l |
| 72 kg | 2,0 l |
| 81 kg | 2,25 l |
| 90 kg | 2,5 l |
| 99 kg | 2,75 l |
| 108 kg | 3,0 l |
| 117 kg | 3,25 l |
| 126 kg | 3,5 l |
| 135 kg | 3,75 l |
| 144 kg | 4,0 l |
Water - is strong solvent. What do you think, at what temperature water weighs more? The highest density of water is at a temperature of 4 0C, above and below this temperature the density is gradually decreasing. Accordingly the water weighs most with 4 0C.
Distilled water to taste has difference from common water. This is because common water has dissolved different salts ,- it calcium salts (presence of these salts complicates the formation of soap when washing), magnesium (usually gives the bitterness water), also iron salts, salts of alkali metals and many other substances. Water enriched metal ions, useful for the organism (but not from the water tap!). For example, potassium and magnesium for the work of the heart muscle, calcium and iron for blood coagulation, sodium for forming mineral salts, with alkaline reaction and is able to decompose organic substance.
Everyone knows that the human body consists of 81% of water and, of course, constantly needs to be replenished. Therefore, the rate of water intake by the human body depending on the weight of the person:
Water hardness
Water hardness - is determined by the presence of cations of calcium (Ca) and magnesium (Mg) in water. The higher the number of these cations, the more water hardness. There are temporary hardness and permanent hardness of water. Temporary hardness is removed by boiling. Salt, which are dissolved (hydro-carbonates calcium and magnesium Ca(HCO3)2, Mg(HCO3)2) easily are desayed when heated to water and carbon dioxide:
Ca(HCO3)2→ CaCO3+H2O+CO2
Mg(HCO3)2→ MgCO3+H2O+CO2
Permanent hardness boiling is not possible to delete. This water contains salts - sulphates, chlorides, nitrates, calcium, magnesium. But You can try to get rid of permanent water hardness: use the milk of lime (slaked lime - Ca(OH)2) or soda).
One of the most effective way to delete permanent water hardness is the use of sodium orthophosphate (Na3PO4). When it reacts with water, the salts fall out in the water:
Ca(HCO3)2 +2Na3PO4→ Ca3(PO4)2 + 6NaHCO3
3MgSO4+2Na3PO4→ Mg3(PO4)2 + 3Na2SO4
When boiling hardness water, on the walls ware formed a scum - salts of calcium and magnesium. They bad to pass heat. If the scum was formed much, walls of dishes can overheat.
When washing in hard water, soap spent much more. It is because complex substances are formed - stearates of calcium and magnesium (Ca(C17H35C00)2 and Mg(C17H35COO)2)
Vegetables are saved longer in hardness water, because the carbohydrates contained in vegetables, form insoluble complex substances from salts of calcium and magnesium.
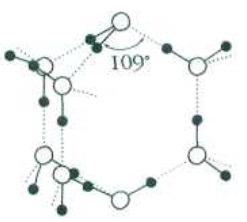
Water and Ice. Why the water is heavier ice?!
Fragments (H2O)8 are saved in liquid water. A number of same water molecules inside of such units H2O, therefore the density of water is higher than the density of ice (900 kg/m3)
About running drinking water:
Everyone knows, that the clearance of water is produced using bleach or solution containing chlorine. Microorganisms cannot survive and die in such water.
But it gets worse water quality, because chlorine is a very powerful oxidizing substance and it reacts with the dissolved impurities and forms organochlorine compounds that are harmful to health.
Dioxins are the most toxic and harmful substances. These substances are the real poisons!
They take a damage affect for human organs, disrupt their proper functioning. But some filters can not always to cope with dissolved chlorine.
Of all the water on our planet only 1% is suitable for drinking. Although there is a great variety of different cleaning filters, even the best filter (layers containing ion-exchange environment, activated carbon) is not able to eliminate all toxic substances from the water. To get clean water You can regular filter replacement (cartridge), also You should know the source from which water is taken and how cleared (for example, in highly polluted areas, the filter should be changed more often).
The clean and healthy water is obtained from natural sources, which is pre-processed, additionally purified and bottled in glass bottles!
Snowflakes
Snow forms when microscopic water droplets in clouds are attracted to the dust particles and freeze. Ice crystals, that appear, do not exceed the first 0,1mm in diametersize, falling down and growing due to the condensation of moisture from the air. This movement formed hexagonal crystal form. Through the structure of water molecules between the tops of the crystal angles formed angles sizes only 60 and 120 degrees. The main water crystal is in the horizontal plane has form of a regular hexagon. Small new crystals settle on the tops of the hexagon, new small crystals form new and new various forms of stars - snowflakes.

t sufficiently high temperatures crystals repeatedly melt and crystallize again. This process breaks the right shape of snowflakes and forms mixed varieties. Crystallization of all the six corners of snowflakes occurs simultaneously in almost the same conditions and, therefore, form the corners of snowflakes get also equally.
To the point of crystallography most natural form of snowflakes is "hexagonal" symmetry. But sometime You can see triangular snowflakes in nature. The reasons for such differences still remain unknown.
For getting triangular snowflakes necessary temperature of 2 degrees below zero.
American physicists studied the effect of various factors on the growth snowflakes. According to physics theory, there are 2 major factors: dynamics of diffusion molecules in the air and dynamics of the behaviour of these molecules on the crystal surface.
They determined that these settings are directly are determined by the air flow, which cover a snowflake.
Scientists checked heir theoretical assumptions with special "snow machine" camera, which can be used to control growth of snowflakes. n result it was determined that the triangular snowflakes are the most persistent, that is, the change of air flow does not change the shape of snowflakes. This, according to scientists, explains the spread of triangular snowflakes.
Recently, scientists from the United Kingdom managed to get the "Pentagon" snowflake. They put a thin layer of ice on the surface of copper. In this layer the molecules of water were located at the tops of the pentagon