Nonmetals





>Nonmetals - are simple substances. Typical nonmetals include gases and liquids. Nonmetals unlike metals much worse conduct electric current, have differences physical-mechanical properties and the transition temperature in the aggregate state.
However, some non-metals have strong metallic properties - Shine (for example, crystalline sulfur, crystalline iodine, carbon, etc.).
In the periodic table metallic properties simple substances (or chemical elements) decreases from left to right, and non-metallic properties increase. By its nature, this can be explained by the change (increase!) the number of electrons on the last orbital level (also called valence electrons). The more pronounced nonmetallic properties of the chemical element, the it "easier" comes in chemical reactions with typicalmetals, filling its last orbital missing electrons and forming strong (from the point of view of chemistry) chemical compounds.
Chlorine
Typical nonmetals are gases. They do not show similar metals properties under normal conditions.
chlorine (CL2). Under normal conditions chlorine is a yellowish-green gas with a pungent suffocating odor. Chlorine is very toxic even at such a low concentration as 0.001 mg per 1 DM3 air. This gas is 2.5 times heavier than air, so will always be placed near the ground in the form of yellowish-green fog.
When the chlorine content in the air of 0.9 ml/l death comes within five minutes. In small quantities (we feel chlorine already in the concentrations in the air of 0.003 ml/l), it is highly irritating to mucous membranes of the respiratory tract and cause coughing.
Chlorine does not interact directly with oxygen, nitrogen, carbon and inert gases. The oxidative properties of chlorine are manifested in reactions with simple substances (metallic natrium Na, iron Fe, phosphorus P sulphur S) and some complex substances.
Chlorine is contained in the earth's crust by weight of 0.017%. In some mountainous areas it covers the base of the soil and therefore leads to the death of insects, small rodents and microorganisms. The boiling point of chlorine is equal to (-33.6 °C) and melting point (-100,98 °C)
Chlorine refers to a number of halogen (fluorine F2, chlorine Cl2, Brom Br2, iodine I2, astatine At2 ), which gives it some features in chemical reactions. It replaces any of the Halogens in reactions with other halogens, standing after him (Brom, iodine and astatine).
chlorine Gas perfectly soluble in cold water, 1 volume of water dissolves about 2 volumes of chlorine to form two acids: hydrochloric acid (HCl) and hypochlorous (HClO), the latter is not stable and decomposes into atomic oxygen and hydrochloric acid. The resulting acid is one of the strong acids.
Chlorine has an excellent disinfectant property. It destroys almost all living organisms in the sphere of its influence, which makes it useful in medicine and household (powders, bleach is the same chlorine water, soda chlorinated). Dry chlorine has not such ability. Chlorine has an excellent disinfectant property. It destroys almost all living organisms in the sphere of its influence, which makes it useful in medicine and household (powders, bleach is the same chlorine water, soda chlorinated). Dry chlorine such ability has not. Chlorine Solution in water is called "chlorine water" which has an excellent property of bleaching materials (fabric, wood, paper etc). The chlorine solution "destroys" the paint on clothing, so if you do not want your blouse or shirt was covered with white spots - do not use this water when washing colored clothes. Just put the wet dyed fabric in a stream of chlorine, as soon it will lose its color and turn into pure white.
As chlorinated water affects to hair, for example, when bathing in the pool (can be found on page Composition of shampoo).
Obtaining chlorine
Chlorine is a poisonous gas that is heavier than air, so the reaction it accumulated on the bottom of the flask.
For chemical reactions, we need potassium permanganate (potassium permanganate) and hydrochloric acid.
In one flask sprinkle a little potassium permanganate, attach the tube for removal of chlorine, which will be released when the reaction of potassium permanganate with hydrochloric acid. The other end of the tube drop into an empty flask (it is to not inhale!). Desirable such design as the picture on the left. If everything is ready, start:
Add hydrochloric acid to potassium permanganate. Watch highlight yellow-green gas, it is chlorine. The reaction proceeds as follows:
2KMnO4+16HCl=2KCl+2MnCl2+5Cl2+8H2O
Substances that are formed during the reaction (except chlorine) - 2 salts - chloride KCl potassium and chloride manganese MnCl2 and water.
fluorine
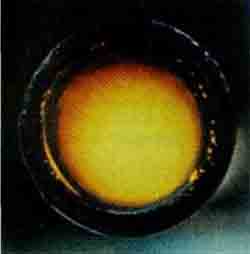
FluorineF2 — bright-yellow gas with an orange tint (t PL -220 °C, t Kip -188°C). About true color fluorine there were a lot of differences: due to the unusually high reactivity of the few who dared to get this gas in sufficient quantities in a transparent vessel. But subsequent studies have confirmed the color of fluoride.
Fluorine interacts with almost all the simple substances, including heavy inert gases (Kg (krypton), Xe (xenon)). Fluorineas chlorinerefers to the number of halogens.
Bromine
Бром Br2 — volatile liquid dark red (t melting -7 °C, t boiling + 59 °C), soluble in water (at 20 °C dissolved 3.6 g of bromine (Br2) in 100 ml of water and organic solvents. A pair of bromine is highly toxic. Burns bromine is very painful and does not heal long tome. If Brom or bromine water gets on the skin, you should immediately wash the burn with large amounts of water, and then with a solution of baking soda, which neutralizes the bromine.
.
Iodine

Iodine (I2) (Tmelt=114 °C, boiling point=185 °C) is familiar to everyone since childhood: 5% water-alcohol iodine solution is used for disinfection of wounds and cuts. If you pour the iodine solution in a porcelain cup and leave for a few hours, the alcohol will evaporate and will stand out crystalline iodine in the form of grey crystals with a metal brilliance, soluble in organic solvents. The heating is a small crystal of iodine sublimates, forming a pair of purple color.
As chlorine, fluorine, bromine, iodine - also belongs to the Halogens. In the normal state iodine - dark gray crystals with metallic luster. In this way it is possible to melt, heated to a temperature to 133.5°C.
Crystalline iodine is not dissolved in water, and soluble in alcohol well. Alcohol solution of iodine has a brown color (it is sold in pharmacies in the form of a 5-10% solution of iodine). Pairs of iodine have a dark purple color.
Iodine is a great preventative tool against radiation. Add to food contributes to strengthening the thyroid, which is more susceptible to radioactive impact. For uptake of iodine by the body in food use iodized salt (KI - potassium iodide). This salt as additives in table salt (NaCl) can be purchased in food shops.
Interestingly, the reactivity of iodine in the "colored" solutions (purple and brown) - varies. So, in brown solutions of iodine is much more active than in purple, for example, reacts faster with copper. This is because iodine molecules can interact with the solvent molecules, forming complexes, in which iodine is more active. It is the solvent has a main role in the manifestation of the activity of iodine!
Adding vegetable oil to the solution of iodine you can watch the transition of the iodine from the aqueous phase to the organic (extraction). The extraction process will be much faster if the mixture shake.
Something about the benefits of iodine:
Iodine is one of the very important elements for human body. The normal dose for human consumption is measured in micrograms, but its absence in the body is dangerous to human life. Iodine is involved in the synthesis of thyroid hormones, which, in turn, are responsible for normal growth of the human body; iodine also responsible for the proper operation of the brain!