Natrium

Natrium (Na) - alkaline metal. Natrium, when dissolved in water, forms one of the strongest alkalis - Natrium hydroxide. Dissolution in water occurs with the rapid release of heat and gas (hydrogen). By hands metallic sodium to take is not desirable - you can get burned!
Sodium, like other alkali metals, it is difficult to get in the laboratory, although it can be done, for example, strongly heating the soda with coal:
Na2CO3 + 2C → 3CO + 2Na.
In industry, the natrium produced with electrolysis of molten mixture of the chlorides of natrium and calcium (a mixture melts at a lower temperature than pure natrium chloride). Interestingly, the first from the melt is not calcium metal but more active - natrium, as the ion Na+ in the melt is lighter than the ion of Ca2+, accepts electrons. The process is carried out in a steel cell at 580 0C. The resulting liquid sodium floats on the surface of the melt and is collected in a special receiver. Each year the world produces about 200 thousand tons of sodium, which is used in nuclear power plants and in aircraft engines as a coolant, in metallurgy as a reducing agent.
lithium

lithium Metal (Li), as natrium, refers to alkali metals. In the free state of lithium is a silvery white metal, soft, although harder than other alkali metals. Lithium, like natrium, is a low-melting metal. Its melting point 181 0C. Lithium is so light (the density of lithium is 0.53 g/cm3) that does not sink even in kerosene.
Natrium, lithium, potassium etc. alkali metals color the flame a characteristic color. Thus, the presence of lithium ions in matter gives the flame red, sodium - yellow, potassium violet, rubidium and cesium is also purple but a lighter shade.
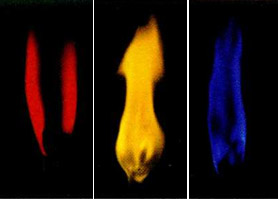
Color of flame with alkali metal ions:
lithium (red), natrium (yellow), potassium (violet)
Compared with other alkali metals lithium has a number of features. Thus, when burning in air it gives the oxide Li2O, and communicates directly with nitrogen to form lithium nitride, Li3N, and with carbon to form lithium carbide Li2C2. Some lithium salts (lithium carbonate, lithium fluoride) slightly soluble in water, and carbonate and lithium hydroxide decompose with strong heating with the formation of lithium oxide. All these properties indicate that the chemistry of lithium is close to the chemistry of magnesium (Mg).
Titanium

Titan (Ti) is a silvery white metal, melting at 1668 0C. Titanium has incredible plasticity - it is possible to get a thin foil. On the strength of the titanium is superior to pure iron (at half density), and its corrosion resistance exclusively above - titanium metal can be stored in sea water. Due to such remarkable mechanical properties, titanium is an indispensable construction material. Titan is the basis of most of the alloys used in shipbuilding, aviation, aerospace and nuclear engineering.
Amazing chemical resistance titanium. Often it is higher than that of noble metals. Titan, for example, not react with chlorine water, a mixture of concentrated nitric and sulphuric acids, and even Aqua Regia (gold dissolves in all of these fluids). The reason is that already under normal conditions on the surface of titanium forms a protective oxide film; under the action of oxidising agents it becomes even thicker and stronger.
Excellent resistance titanium for corrosion. If in sea water put the plate of pure aluminum, monel (copper-nickel alloy used for minting coins), stainless steel and titanium with a thickness of 1 mm, their fate will be different. Aluminum plate after a few days covered with gray spots (pitting), and five months later, is destroyed. Monel will become dark green due to the reaction copper and nickel with aggressive sea water, and about a year later it will suffer the fate of aluminum. Steel plate will last about four years, gradually covered the rusty spots. By the way, shells and seaweed significantly accelerate the destruction of the steel. Titanium disc even in a thousand years (!) will remain almost unharmed: corrosion penetrate it only in 0.02 mm. In this reactions, the titanium for durability not inferior to platinum.
Although durable the oxide film protects the titanium from oxidation, it is quite readily soluble in concentrated hydrochloric and hydrofluoric acids:
Ti + 6HF → H2TiF6 + 2H2;
2Ti + 6HCl → 2TiCl3 + 3H2
Titan has its own "Achilles heel" - he is very "afraid" of the compounds of fluorine (F). In hydrofluoric (hydrofluoric) acid titanium dissolves as fast as magnesium reacts with hydrochloric acid.