The rate of chemical reaction is the change of the concentration of substances, which entered into a chemical reaction (i.e. the change in concentration of reactants). In practice, most often it is measured in moles per liter (mol/liter) per unit time (second, minute, hour).
Rate of chemical reaction - it is not a constant value and is measured exactly at a given point time. It is because in the process of chemical reaction, the molecules of one substance combine with other molecules and it is reducing the probability of collisions of the original molecules of the reagent. Accordingly, the lower molecules of the reactants, the less they combine. Therefore, the rate of chemical reactions are largely influenced with the initial concentration of the reactants.
The rate of chemical reaction can be calculated. The formula may be written as:
v=k[A]a[B]b,
k - is a constant value (constant of rate), A and B - are substances, entered into a chemical reaction, a and b - are numbers indicating partially the reaction order.Coefficients of the equation get experimentally, then if the theoretical model coincides with the practical, the theoretical model is used for the calculations.
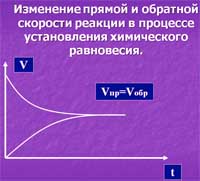
The dependence of the rate of a chemical reaction from time of chemical reaction
What is the rate of chemical reactions depends on?
So, one of the conditions of rate reaction can serve as a concentration of the reacting substances. Other factors that affect to the rate of chemical reactions are: temperature, presence of catalysts, in some reactions most important affect for rate reaction is the presence of ultraviolet light or simply daylight. So with the influence of temperature the kinetic energy of the molecules increases, they begin to move faster, and the probability of collision increases! Catalysts also have a positive effect on the rate of chemical reactions, increasing the area of contact between molecules of the reacting substances. The light speeds up the chemical reaction, for example, separation of crystals of silver on the photo paper (reduction reaction of silver), the reaction of photosynthesis in plants also occurs at the action of light. The reaction rate rapidly decreases when the consumption of the reagents (or one of them). Sometimes are used inhibitors of chemical reactions (substances that slow the rate of chemical reactions).
In addition to the main reaction - a direct, is a parallel (reverse) chemical reaction, because the reaction products can react with each other. In the initial moments of the reaction the concentration of reactants is large, therefore, first occurs in the main direct reaction. Over time, increases the concentration of the reaction products and these products react among themselves with the formation of reagents. This process can be repeated many times until the rate of chemical reactions (forward and reverse) become equal. At the level of molecules, reaction does not stop.