Natural soap
What is a natural soap?
Soap - it is a salt of alkali metals and higher carboxylic acids, that contains from 10 to 22 carbon atoms in the chain.
Before the invention of natural soap grease and dirt from the skin was removed by ash and fine river sand. The Egyptians washed mixed with a water-based paste beeswax. In Ancient Rome when washing were used powdered chalk, pumice, ash.Apparently, the Romans did not confuse that with such ablutions along with dirt could "scrape" and part of the skin. The invention of natural soap is probably the Gallic tribes. Galls did ointment from fat and ash beech tree, which is used for hair coloring and treatment of skin diseases. And in the second century it was used as a detergent.
The technology of making natural soap from animal fats evolved over many centuries. First is composed of fat mixture which is melted and omelet - boiled with lye. The formation of natural soap (salts of fatty acids) and glycerinate. Glycerin and pollution from the reaction mass is then precipitated by adding sodium chloride. In the soap mass is formed of two layers - the core (neat soap) and podmienky liquor.Pure soap is separated, cooled, dried and packed.
For a long time in the composition of natural Soaps included only waste from the processing of animal fats. In 1843, in the German made natural soap of high quality white fat with the addition of a new component of coconut oil. At first it was poorly sold because not had the usual for that time of repulsive smell of rancid fat, and therefore, it was poorhe opinion of the buyers. Later, a new product has received well-deserved recognition, and since then, the classic natural soap are included sodium salts of fatty acids of coconut oil and beef fat in the ratio 1:4.
The cleaning action of soap is due to the structure of salts of fatty acids. Their molecules consist of two parts with different affinity for water (hydrophilic carboxyl group and the metal ion) and hydrophobic (hydrocarbon radical). When washing the hydrophilic part of the molecule of natural soaps turned toward the water, and hydrophobic (nonpolar) hydrocarbon "tails" are immersed in fat droplets.
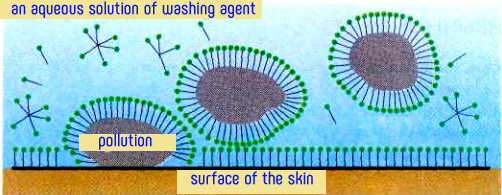
Due to this "two faces" soap solution wets well the surface with hydrophobic contaminants. Soap film is formed between the surface of the skin and impurities, which appears due to the coupling the dirt with skin and facilitates their transition into the cleaning solution. Thus, soap behaves as a surface-active substance (surfactant). As salts of fatty acids form in the solution of surface-active anions RCOO-, these compounds are anionic surfactants.
Now a huge number of other cosmetic cleansers. This liquid soap, and shampoo, and shower gel and foam bath.
What supplements would not contained in the soap, what would be beautiful it has not been transparent, colored, patterned, aromatic) - in any case, the natural soap is salts of fatty acids (palmitate and sodium stearate - for solid soap). Such chemical compounds (salts of fatty acids) are easily dissolved in water, of course, if formed from one of the alkali metals (sodium, Na or potassium (K).
One of the widely used fatty acids - stearic acid (C17H35 - COOH)and its salts - stearates (for example, sodium stearate17H35 - COONa) - soluble in water.
The stearates of magnesium, calcium stearate or stearate iron or even stearic acid - a substance insoluble in water. This property is used for water softening: if you add soda ash to hard water, that is containing dissolved salts of magnesium and calcium, you can easily soften the water, because are formed composition water-insoluble calcium carbonate or magnesium.
As already mentioned, natural soap receive, act on the fat and alkali. This reaction is called a chemical reaction of saponification, in which it turns out natural soap.
С3H5(OCOС17H35)3 + 3NaOH → С17H35 - COONa + С3H5(OH)3 .
When saponification reaction turned out to be already known sodium stearate (C17H35 - COONa) and not less well-known polyhydric alcohol - glycerine (С3H5(OH)3).
Polyalloy is formed by dissolving the soap in the water, in which are available soap molecules and molecules are grouped into colloidal particles - micelles. At low concentrations of soap in solution, the molecules move freely independently of each other. Micelles are begining formed at low concentrations of soap in solution.
Many of properties the natural soap, such as hardness, solubility, foaming, cleaning ability depends on its composition of added fat.
So, part of beef and pork fat palmitic acid gives a natural soap hardness and good foaming qualities, and oleic acid - solubility in cold water and detergent capacity. Stearic acid enhances the cleaning action of soap in hot water. Due to the lauric acid, that is contained in coconut oil, natural soap dissolves better in cold water, increases its cleaning ability and decreases the swelling; but this acid can cause to skin irritation.
But because of linoleic acid (component lard) natural soap unpleasant smell and becomes unsuitable for long-term storage. Therefore, the content of pork fat in the fat used for the making of soap, usually small.
In addition in the soap also introduce various additives. This filler (titanium oxide or zinc), perfume, dyes, humectants (glycerin, castor oil, waxes of animal origin such as lanolin, and spermaceti). Bactericidal and deodorizing natural Soaps contain antiseptic substances such as triclosan (2-hydroxy-2',4,4'-trichlorodiphenyl ether).
So, there are several disadvantages soap:
- soap loses detergent in hard water;
- soap is alkaline and due to hydrolysis, and the hydroxide ions destroy fiber fabrics.
I guess that's all. But synthetic detergents has not its , which are sodium salts of sulphonic acids or salts of acidic sulfates of high molecular alcohols. They keep detergency in hard water and less destroy the fabric.
The main disadvantage of synthetic detergents is that they are in the process of their use are not destroyed. Entering the waste water they pollute the environment.