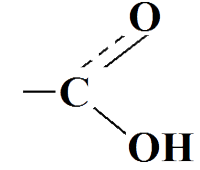
(written - COOH)
Carboxylic acids
Carboxylic acids (or organic acids) are derivatives of hydrocarbons, that have a carboxyl group (- COOH) in molecules.
All carboxylic acids are organic compounds. A typical example is acetic acid - CH3-COOH, formic acid CHOOH, propane (or propionata) CH3-CH2-COOH, oil (or butane) CH3-CH2-CH2-COOH acid.
Properties of organic acids Chemical properties of organic acids is determined by the structure of the functional group (carboxyl group), and these compounds are easily dissociated in solutions with the formation of hydrogen ions. Therefore, for carboxylic acids is characterized by all of the same properties as that of mineral acids.
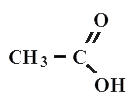
Acetic acid
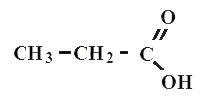
propionic or propane acid
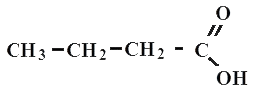
butane or oil acid
Properties of organic acids
- reaction with some metals (we have salt and hydrogen)
CH3-CH2-COOH + K → CH3-CH2-COO-K + H2
- reaction with metal oxides (we have salt and water):
CH3-CH2-COOH + MgO →Mg(CH3-CH2-COO)2 + H2O
- reaction with salt (a different salt is formed and more weak acid):
CH3-CH2-COOH + Na2CO3 → CH3-CH2-COO-Na + H2O + CO2
- reaction with alcohols (formation of ester):
CH3-CH2-COOH + CH3- OH →(в присутствии H2SO4) -> CH3-COO-CH3
- reaction with halogens (we have a halogenated acids)
Getting an carboxylic acids
Getting an carboxylic acids from aldehydes, that are got by oxidation of alcohol. The reaction proceeds:
- this is the oxidation reaction of ethanol with oxygen.
- this oxidation reaction of acetic aldehyde with oxygen.
Getting an carboxylic acids hydrolysis nitriles: the reaction takes place with the action water to nitriles. The second product of the reaction is ammonia:
In addition, in this reaction (intermediate product) is formed of a highly unstable triatomic alcohol, that has a molecule with three hydroxyl groups at only one carbon atom.
During the hydrolysis of esters we also get carboxylic acids. The Second product of it reaction is alcohol. To shift the equilibrium of the reaction can use lye, but this way we will get not carboxylic acid, but its salt. If the salt is treated with a strng mineral acid (e.g., hydrochloric), we get a carboxylic acid.
This method of getting an carboxylic acids is used mainly to get higher carboxylic acids: palmitic and stearic - from fats of oils.
Lower carboxylic acids are readily soluble in water and dissociated. But it is important to note, that the larger molecular weight (i.e., the more atoms included in a molecule of acid), the solubility in water decreases. This is due to the increasing size of the hydrocarbon radical. So, higher carboxylic acids (e.g., stearic) do not dissolve in water. But, all acids are readily soluble in alcohol and ether.
The presence of hydrogen ions in solutions of carboxylic acids, like other acids, you may use the indicator. The carboxylic acids, as mineral acids, react with metal oxides, bases, salts. In reactions with salts esters are formed.
Stearic acid

Stearic acid
All probably know stearic acid. This substance, insoluble in water, gray color (think about candles!).
It is a weak acid. Stearic acid can be got with acting on the soap solution with any other acid. For example, if to soap solution add acetic acid, it is possible to observe gray precipitation, it will be stearic acid:
C17H35COONa + CH3COOH => C17H35COOH (precipitation) + CH3COONa
In this reaction C17H35COONa - solution of solid natural soap (for wash hands). In chemical terms the soap is sodium stearate. Similarly, other salt of stearic acid are called stearates.
C17H35COONa + CH3COOH → C17H35COOH (осадок) + CH3COONa
В этой реакции C17H35COONa - раствор твёрдого натурального мыла (им моем руки). На химическом языке твёрдое мыло - стеарат натрия. Аналогично этому, другие соли стеариновой кислоты называются стеараты.
Lemon acid

Lemon acid (chemical formula is HOOCCH2)2C(OH)COOH) forms large colourless, transparent crystals, soluble in water and in ethyl alcohol. Salt of lemon acid (citric acid) are called citrates.
Most often citric acid is used as flavor additives for confectionery products. When mixing citric acid and baking soda (NaHCO3) we get carbon dioxide (especially when heated), that loosens the dough.
Citric acid (lemon acid) can also used for removing ink stains and rust.
Salt - citrate of iron - colorless substances, are soluble in water. This property is used for removing rust spots.