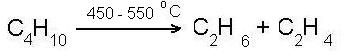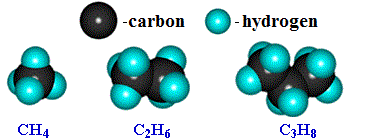
Saturated hydrocarbons - their name speaks for itself! It is a complex chemical substance, whose molecules are composed of hydrogen atoms and carbon atoms.
Saturated hydrocarbons form a homologous series of hydrocarbons, each chemical substance in this row differs from the previous substance on same number of atoms of hydrogen and carbon in it structure.
Homologous series of hydrocarbons
- CH4 - methane
- C2H6 - ethan
- C3H8 - propane
- C4H10 - butane
- C5H12 - pentane
- C6H14 - hexane
- C7H16 - heptane
- C8H18 - octane
- C9H20 - nonane
- C10H22 - dekan ...
Homologous series of hydrocarbons is characterized with the formula, that can determine the formula of any substance a it series. For hydrocarbons it is formula
CnH2n+2
With the increase of molecular weight of the substance (number of atoms of carbon and hydrogen in the molecule are increasing) increases the boiling point of the substance. Therefore, it should be noted that substances with CH4 to C4H10 are gases, next C5H12 to C15H32 are fluids and next are solids!
Saturated hydrocarbons have some title - waxes.
Saturated hydrocarbons form an aisomers are substances that have the same number of atoms and the same elements but have different spatial structure. For example, a group of CH3 (or methyl) may connect with the first or with the second, or the third atom of hydrogen.
Isomers are typical for saturated hydrocarbons, since C4H10 - butane.
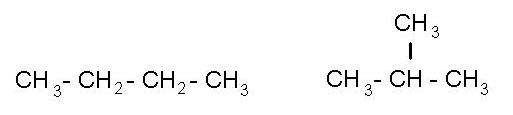
To the left - formula of gas - butane, to the right is the same Bhutan, but only in form of isomer. If to detach one atom of hydrogen from saturated hydrocarbon then get a very reactive chemical substance that practically does not exist in the free state and instantly reacts.
Saturated hydrocarbons at room temperature (all the alkanes) are inert in chemical behaviour (i.e., does not enter into chemical reactions), but at higher temperatures get a chemical activity.
Properties of saturated hydrocarbons
- substitution reaction: (reaction of halogenation)
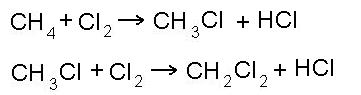
- reaction of nitration:
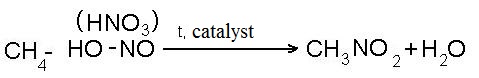
- reaction of dehydrogenation (removal of hydrogen)
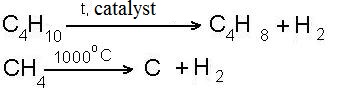
- combustion reactions
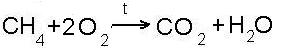
- cracking reaction (splitting of large molecules into smaller molecules)